Methanotrophic bacteria consume 30 million metric tons of methane per year and have captivated researchers with their herbal ability to convert resistant greenhouse fuel into usable fuel. However, we know very little about how complex reaction occurs, which limits our ability to use double merit to our merit.
By reading the enzyme used through the bacterium to catalyze the reaction, a team at Northwestern University has discovered key structures that can drive the process.
Their discoveries may eventually lead to the development of man-made biological catalysts that convert methane into methanol.
“Methane has a very strong bond, so it’s pretty remarkable that there’s an enzyme that can do that,” said Amy Rosenzweig of Northwestern, leader of the paper. “If we don’t accurately perceive how the enzyme plays this complicated chemistry, it might not be able to design and optimize it for biotechnological applications. “
The enzyme, called particulate methane monooxygenase (pMMO), is a particularly complicated protein because it is embedded in the bacteria’s mobile membrane.
Typically, when researchers examine those methanotrophic bacteria, they use a rigorous procedure in which proteins are extracted from mobile membranes with a detergent solution. While this procedure isolates the enzyme well, it also eliminates any enzymatic activity and limits the amount of data researchers can collect. such as tracking a center without a central heartbeat.
READ MORE: This Selling Device Refills Cleaning Products: Restrict Plastic and Save Money
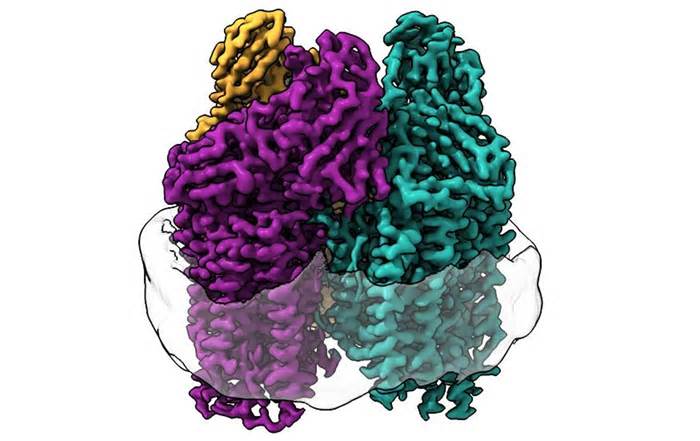
“By recreating the local environment of the enzyme in the nanodisk, we were able to repair the activity of the enzyme,” Koo said. “We were then able to use structural techniques to determine how the lipid bilayer repaired activity at the atomic point. discovered the entire arrangement of the copper site in the enzyme where methane oxidation is most likely to occur.
The researchers used cryoelectronic microscopy (cryo-EM), a very suitable strategy for membrane proteins because the lipid membrane environment is not altered during the experiment. This allowed them to visualize for the first time the atomic design of the active enzyme at maximum resolution. .
MORE: BeyondPlastic Awards for Cutting-Edge Packaging: Bags That Melt When Boiled and a Sustainable Toothpaste Tube
“As a result of the recent ‘resolution revolution’ in cryo-EM, we were able to see the design in the atomic details,” Rosenzweig said. “What we saw absolutely replaced the way we think about the active site of this enzyme. “
Rosenzweig said cryo-EM structures provide a new starting point for answering questions that continue to pile up. How does methane travel to the active site of the enzyme?How does copper carry out the chemical reaction from the active site?Next, the team plans to examine the enzyme directly in the bacterial mobile using an imaging strategy called cryo-electron tomography (cryo-ET).
RELATED: Scientists Fight Ocean Plastic with Biodegradable Flip-Flops Made of Algae
If successful, researchers will be able to see precisely how the enzyme is organized in the mobile membrane, figure out how it works in its actual local environment, and whether other proteins around the enzyme interact with it. These discoveries would provide a missing key link for engineers.
“If you optimize the enzyme to connect it to biomanufacturing pathways or to consume pollutants other than methane, we want to know what it looks like in its original environment and where the methane binds,” Rosenzweig said. “You can use bacteria with a changed enzyme to collect methane from fracking sites or to plug oil spills. “
This study is published in the journal Science.
Data Source Provider: Northwestern University
SHARES this groundbreaking research; Share the hope. . .
Want a morning jolt of news?