When Pfizer announced last week that his COVID-19 vaccine was much higher than expected, I had a reaction.
Of course, like everyone else, I saw this as good news, but also a bit like the other team scored.
This is because I am one of 30,000 volunteers in a clinical trial of another vaccine developed through a competing company, Moderna.
Leaving aside my main interest, Last Monday’s Pfizer news was a counterweight to the grim statistics that the virus is out of control and spread across the country.
Ironically, these high rates of infection may bring us closer to the end of the pandemic. The more infections among the participants examined, the faster we will know if the vaccine works better than placebo to prevent their receptors from getting sick.
On Thursday, there’s more smart news. Moderna, the Massachusetts biotechnology company I’m enrolled in, said it had also exceeded the threshold of its first interim effects investigation and was preparing knowledge for an independent review panel.
Pfizer’s effects gave the impression that the attack of the “peaks” depicted on the coronavirus surface works. This is fortunate, as other vaccine applicants in the study use the same strategy.
COVID update: New Jersey COVID instances have reached a ‘devastating’ point that has been noticed since the first wave
Holiday Meetings: Want to join as a family circle for Thanksgiving?Experts say quarantine begins now
Better yet, Pfizer’s approach to provoking this immune reaction, by injecting a genetic clothing extract instead of a dead or living virus, also works. pandemic viruses.
But what’s more important to me is the same strategy used through Moderna in the vaccine for which I’m guinea pig. This makes me positive that “my” vaccine will have equally positive results.
I worry more than I did a month ago. That’s because after the time of two injections, I’m pretty sure I didn’t get a placebo.
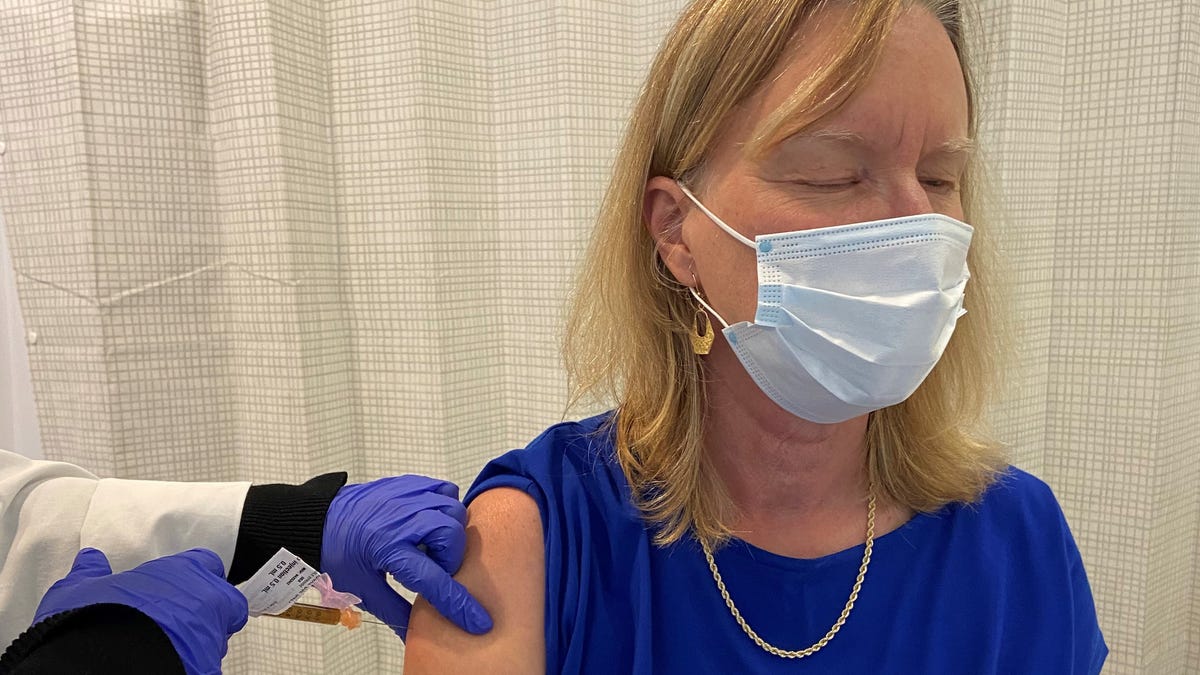
This is a double-blind clinical trial, which means that none of the test leaders at Hackensack University Medical Center, where I enrolled in modern’s COVE exam, knows if the syringe that stuck in my arm contained a placebo or a reality. 30,000 participants in Moderna’s essay, myself included, were informed of the organization to which we belong.
But here’s why I think I got the candidate vaccine: my framework reacted.
After the first injection in September, I had no reaction, apart from a pink trepoint of the length of a mosquito bite on my arm and some stiffness around the injection site. I’m sure it’s placebo.
A month later, the moment the shot entered easily. The nurse who prepared the syringe, with her long needle and the swampy brown cloth cylinder, joked that she had to kill her to find out what was inside.
The principal investigator of the COVE study at Hackensack Meridian Health, Dr. Bindu Balani, asked the doctor to warn volunteers that they could enjoy more pain, in all likelihood fever and headaches after the injection.
It would be soft and transient, said my nurse, “not enough to interrupt her life. I had to record my temperature and other symptoms in a phone app, as I did after the first injection. “
The next morning, my arm was stiff, like the muscles were swollen, and he didn’t need to bend or stretch. During my 8 a. m. appointment. With a private trainer, lifting an 8 pound weight above my head, without any challenge. – It was painful.
Then I was so tired I had to take a nap in the morning. A mild fever, which decreased with ibuprofen, peaked around dinner time at 100. 3. I went to bed early, after what had been a pitiful day.
But that’s it.
Two days after the injection, I felt normal.
Note that I am an anecdote in an exam that involved thousands of participants, but from what I read and said, my reaction is not uncommon.
For me, if that’s all it takes to be from a virus that can kill me, it’s a smart deal.
Will the rest of the country see the things I do?
If these two vaccines are approved, the situations that demand communication and logistics are immense.
First, ask for two injections, Pfizer spaced 3 weeks aside and Modern 4 weeks. Cannot be combined and adjusted; if you start with Pfizer, you must end up with Pfizer.
Second, injections should be sent and stored at sub-zero temperatures. This will require special freezers or dry snow, a shipping “cold chain” that is being worked on through Operation Warp Speed, the federal immunization project.
Finally, there’s the acceptance. A vehement minority of Americans, many in New Jersey, are already expressing skepticism about the need and protection of vaccines; in recent months, others have expressed fear that the progression and approval of the COVID vaccine has has been hasty and politicized. In mid-September, he found that only 50% of Americans were willing to get vaccinated.
A survey of New Jersey doctors and nurses conducted for the Department of Health in mid-October found that two-thirds of doctors expressed a desire to receive the COVID-19 vaccine, while less than a portion of nurses (47%) I would have done it or probably vaccinated.
Add to that the pain factor. Will other people come back for a moment to inject the yourself if they know you can leave them in poor health for a day?What if they have to quit their jobs?
Much remains to be learned about the short- and long-term protection of vaccines. The protective effects of Pfizer and Moderna were not revealed, but they are more complex than Johnson’s studies.
Study participants will be monitored for two months after the injection to check for serious side effects or other adverse occasions before one of those corporations can request emergency approval of the vaccine from the Federal Food and Drug Administration.
So every week, like each and every participant, I get a call from a researcher to let me know about new fitness issues. Side effects like the ones I’ve experienced are not serious.
My weekly touch also asks if I’ve been in close contact with known for having COVID-19.
I got it.
What’s safe? : New Jersey school districts are worried about an increase in COVID after the holidays. Some will be out until January
Second wave: full policy as New Jersey faces COVID wave
But across the country, by the end of October, seeing my nonagenary parents (two flights, two hotels, a rental car and a lot of food to take in combination inside their home) would possibly have tested my precautions and my possible immunity. at the moment, I have no symptoms.
Full FDA approval, unlike an emergency use authorization, occurs after several years of follow-up to see if the inoculation of a giant population causes more protection problems, but production can begin after emergency authorization.
For Pfizer, this two-month follow-up era for the maximum of its participants ends this week.
If no protection issues have arisen, and the company’s press release indicates that this has not been the case, Pfizer may apply for permission to use the vaccine.
Plants are able to produce millions of doses, thanks to Operation Warp Speed. If the calendar continues, the first organization of precedence, fitness personnel, can begin to be vaccinated before the end of the year, according to public fitness officials.
Approval of other vaccines can be rapid.
Modern, my vaccine manufacturer, wants about a month of additional follow-up knowledge to meet FDA emergency use authorization requirements. If the Independent Data Surveillance and Security Board to which the knowledge sends discovers that 60% or more of Moderna’s COVID instances belong to the placebo group, Moderna may also request such authorization, as this indicates that the vaccine has reduced COVID instances in the group. who won the vaccine instead of placebo.
On the other hand, if its effects show about the same number of instances in each group, indefinite advice could simply end the trial, Politico reported. And if the knowledge is inconclusive, the exam can continue until more instances of COVID are collected: 106 instances to make the time of the interim investigation and 151 to complete the exam.
But what those vaccines might not do is as vital as they can do. None of them are a quick fix.
Even at 90% efficacy, as announced through Pfizer, one in 10 vaccinated people exposed to the virus will still get sick, and it will be several months before a vaccine becomes widely available to the public.
We still can’t throw away our mask.
So many unanswered questions. Among them:
In an effort to build confidence in the studies and approval process, corporations have released more data than they do about their testing protocols, but those and other questions want to be answered if other people want the data they want for their vaccination . decisions.
As for me, in a few days I’ll go back to Hackensack for more blood samples, they’ll check for COVID-19 antibodies, I’ll never know the results, but I hope I have many.
However, I will return to the same position where I had the first shot.
The studio workplace moved from its original location to the hospital. With COVID on the rise again, the area needs to be able to treat the growing number of patients with the virus that drove this billion-dollar race for a vaccine.
Lindy Washburn is a senior fitness reporter for NorthJersey. com. To stay on top of changes in the medical world in your fitness and that of your family, subscribe or activate your virtual account today.
Email: washburn@northjersey. com
Twitter: @lindywa