“o.itemList.length” “this.config.text.ariaShown”
“This.config.text.ariaFermé”
SPRINGDALE, Arkansas, August 31, 2020 / PRNewswire / – NOWDiagnostics, Inc. today announced that it is running with BARDA, the branch of the Advanced Biomedical Research and Development Authority of the U.S. Department of Health and Human Services.To expand a SERological control of SARS-CoV-2 Antibody that can be used in a variety of physical care settings, from clinics to hospital emergency rooms, and in the end through consumers for home use.
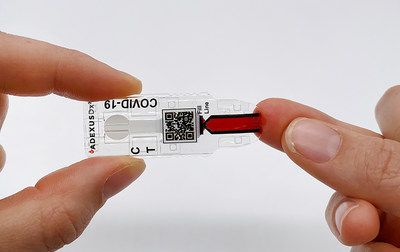
Immediate control of COVID-19 antibodies, the AUTONOMOUS platform ADEXUSDx® of NOWDiagnostics, requires no more appliances (reagents and tampons), appliances, processing or refrigeration.No matter where it is performed, THE ADEXUSDx® COVID-19 control is designed to provide laboratory effects in 15 minutes by detecting the presence of ANTI-SARS-CoV-2 antibodies in others who have been exposed to the virus.-19, if you have had severe, moderate, mild or no symptoms.
The rapid result ADEXUSDx®COVID-19 check requires only one finger to discharge 40 L of capillary blood, venous blood in general, serum or plasma and has the possibility to be implemented directly in homes and workplaces.Service controls (POCs) and over-the-counter (OTC) would allow Americans to be evaluated seamlessly and quickly, resulting in control effects in minutes that days.An Application for Emergency Use Authorization (U.S.) was filed with the Food and Drug Administration (FDA) in May 2020 for the use of moderate complexity of the ADEXUSDx® COVID-19 check.
This allocation was financed as a federally invested component of the Health and Human Services Decompotor; Office of the Under-Secretary for Preparation and Intervention; Advanced Biomedical Research and Development Authority, Research, Innovation and Contracted Companies Division No.75A50120C00156. THE BARDDs for this assignment include a $695,500 investment in allocation and technical prices required for NOWDiagnostics to apply for emergency use authorization from the U.S. Food and Drug Administration.(FDA) for THE POC and OTC uses of the ADEXUSDx® COVID-19 test.
The data contained in this press release is provided through NOWDiagnosiss, Inc.and involve federal approval of the company or its products.
NOWDiagnostics, Inc., founded in Springdale, Arkansas, is a leader in state-of-the-art diagnostic verification.Its exclusive ADEXUSDx platform® has a laboratory at your fingertips, which uses a drop of single blood to verify a variety of non-unusual conditions, diseases and ailments, with effects in minutes.By getting rid of the desire to send checks to external labs, NOWDiagnostics products have the chance to slow down the waiting era to check the effects of a few days to a few minutes.in www.nowdx.com.
View media to download media: http://www.prnewswire.com/news-releases/barda-and-nowdiagnostics-partner-to-develop-rapid-covid-19-antibody-test-for-use-point-of – Home Care-301121184.html
SOURCE NOW